As we knew, to change temperature of m kilogram of a material with specific heat in amount of Δθ, it needs Q Joule of thermal energy. From definition of specific heat, we have
:
In other words
:
Which Q (energy) is at Joule, m (mass) at kilogram, C (specific heat) at Joule per kilogram Celsius degree, and Δθ (temperature change) at Celsius. Now to calculate thermal potential energy of a material, we use below equation
:
Which in above equation UQ (thermal potential energy) at Joule, m (mass) at kilogram, C (specific heat) at Joule per kilogram Celsius degree and θ (temperature) at Kelvin degree. For example, one kilogram of steel with 500 Joule per kilogram Celsius degree as specific heat and 37 Celsius degree as temperature (273+37=310 Kelvin degree) :
It has 155 kilo Joules as saved thermal energy which if catch it, its temperature will be absolute zero. As we know, by increasing velocity, the mass of material will be increased too and this amount of mass increase will be calculated from below equation
:
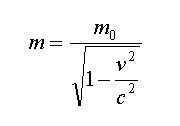
m is mass of on move material, m0 is mass of static material, v is velocity of material movement, and c is light velocity. Now by replacing on move mass in previous equation, the new below equation will be attained in order to check the temperature measure of the on move material
:
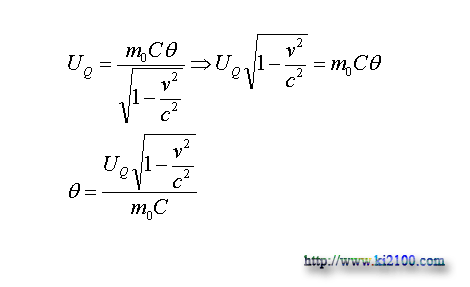
θ
In this equation is the temperature of the on move material that this equation expresses this matter that if the on move material has no temperature exchange with the surrounded environment (its thermal potential energy is consonant), its temperature will be closed to absolute zero with increasing material velocity to light velocity. Because:
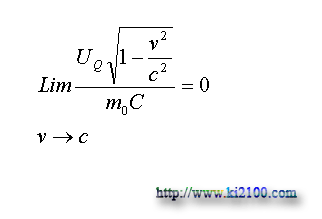
Now we shall draft the equation that shows the relation between temperature of static material and temperature of same material but on move with certain velocity. For this, we put the physical equivalent of UQ for the static material in above equation
:
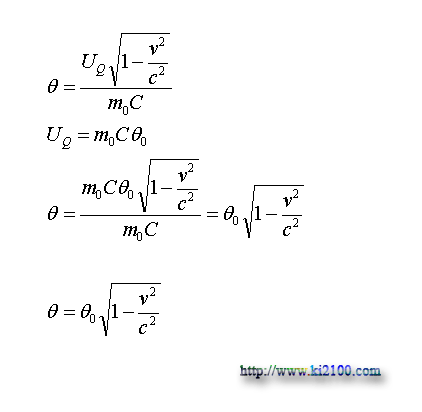
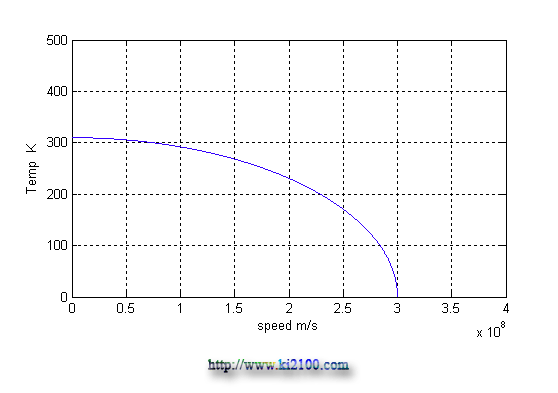
θ0 is the temperature of the static material that finally we reach to a simple equation that is similar to Gerald – Lorentz equation for shortening length and slowing time toward velocity increase. Now we draw the diagram of a material with 37 Celsius degree or 310 Kelvin degree as temperature, when it is closing to light velocity
:
So it seems as closing to light velocity, the material temperature will tend to absolute zero, this will lead to a thermal exchange which surely prevent occurring explosion because of mass and density increase
.
دوست عزیز، به سایت علمی نخبگان جوان خوش آمدید
مشاهده این پیام به این معنی است که شما در سایت عضو نیستید، لطفا در صورت تمایل جهت عضویت در سایت علمی نخبگان جوان اینجا کلیک کنید.
توجه داشته باشید، در صورتی که عضو سایت نباشید نمی توانید از تمامی امکانات و خدمات سایت استفاده کنید.



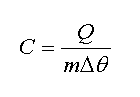
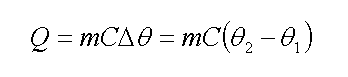
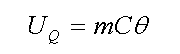
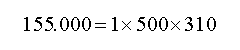



 پاسخ با نقل قول
پاسخ با نقل قول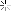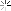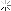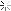


علاقه مندی ها (Bookmarks)